
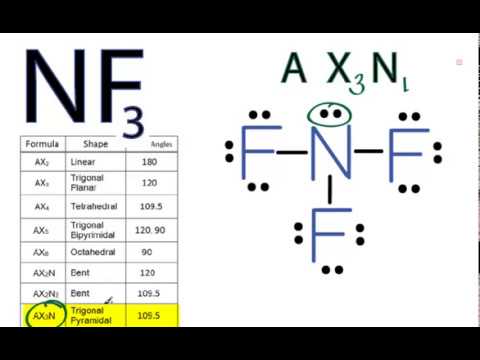
It will use one s and one p orbitals to form the hybrids, and the remaining p-orbitals to form pi bonds with the two sulfur atoms. The carbon atom will thus be #"sp"# hybridized. This means that its steric number will be equal to #2#. In this case, the carbon atom is surrounded by two regions of electron density, one for each double bond it forms with the sulfur atoms. Now, molecular geometry is determined by the hybridization of the central atom. The remaining 8 valence electrons will be placed as lone pairs, two on each sulfur atom.

These bonds will account for 8 of the 16 valence electrons of the molecule. The central carbon atom will form double bonds with the two sulfur atoms. It is made of one Oxygen atom and two Fluorine atoms. The remaining #8# valence electrons will be placed as lone pairs, two on each sulfur atom. Carbon disulfide, CS2, will have a total of 16 valence electrons, 4 from the carbon atom and 6 from each of the two sulfur atoms. In this video we are going to determine the polarity of Oxygen Difluoride having a chemical formula of OF2. These bonds will account for #8# of the #16# valence electrons of the molecule.
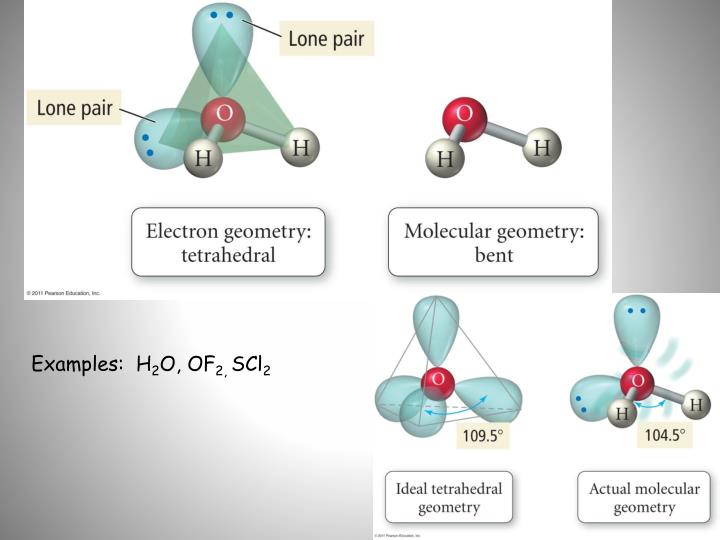
The best place to start when trying to figure out a molecule's geometry is its Lewis structure.Ĭarbon disulfide, #"CS"_2#, will have a total of #16# valence electrons, #4# from the carbon atom and #6# from each of the two sulfur atoms. The molecular geometry and vibrational frequencies of 2- and 5-MB molecules in the ground state have been calculated by using the density functional methods (.


 0 kommentar(er)
0 kommentar(er)
